The global healthcare sector was amazed when a little girl with B-cell acute lymphoblastic leukemia was cured after being treated with chimeric antigen receptor (CAR) T-cell therapies (CAR-T) in 2012.
Dr. Kuo-Hsiang Chuang, who just started his job at the Graduate Institute of Pharmacognosy, Taipei Medical University (TMU) at that time, thought CAR-T would help doctors and patients successfully fight cancer. However, CAR-T, mostly based on retrovirus gene delivery, leaves a lot of room for improvement. Dr. Chuang therefore developed the unique "Armed-T" technology and founded CytoArm in 2020, which now offers five T cell products against different cancers based on the Armed-T cell platform.
CAR-T delivers effective results but has room for improvement
According to a WHO report released on February 1, 2024, there were close to 20 million new cases of cancer in the year 2022 alongside 9.7 million deaths from cancer. It is not an exaggeration to call cancer the biggest threat to human health. This is also why the world including the healthcare sector is paying attention to CAR-T following the successful treatment of the little girl with leukemia in 2012.
According to Dr. Chuang, CAR-T makes use of genetic engineering technologies to change the patient's immune cells called T cells, and enable them to effectively attack the cancer. T cells are collected from the patient and re-engineered in the laboratory to produce proteins on their surface called Chimeric Antigen Receptors (CAR) using retrovirus gene delivery.
The genetically reengineered CAR-T cells can precisely identify tumor-specific antigens. When deployed back in the patient's body, CAR-T cells can specifically bind to cancer cells and destroy them.
FDA approved the first CAR-T therapy in 2017, which has been proven to deliver promising results for blood cancers and impressive survival outcomes. However, Dr. Chuang pointed out the risks of CAR-T. First of all, it uses retrovirus vectors for gene delivery into T cells, which might elicit an unexpected immune response or cause T cell mutation.
Furthermore, CAR-T costs will remain high as the processes of collecting, reengineering, and readministering T cells require a highly specialized team of professionals and expensive lab equipment. The gene (or virus) reengineering and cell culturing processes are also very challenging. Last but not least, CAR-T patents are owned by a small group of big pharma players, barring the entry of newcomers with formidable licensing fees or potential infringement lawsuits.
Armed-T fights cancer even more effectively
CytoArm's Armed-T technology addresses all of the above issues. According to Dr. Chuang, with Armed-T therapy, specific molecules are attached to the surface of the patient's T cell, much like putting armor on the T cell like Iron Man, described by Dr. Chuang.
The armed T cells are then equipped to identify and destroy cancer cells. As opposed to CAR-T therapies that need 30 days for virus culture or reengineering, it only takes 10 days to produce high-purity cancer-fighting Armed-T cells and the therapeutic results are significantly more promising. To top it off, as no retrovirus gene delivery or genome editing technique is used, Armed-T cells are free of cancerization or mutation risks, making Armed-T a safer treatment choice for patients.
In general, it is not easy for genetic reengineering techniques to achieve a high purity. CytoArm's Armed-T technology generates cancer-fighting cells at an impressive 90%+ purity. As to costs, without the need for retrovirus gene delivery, CytoArm can offer Armed-T technology at more affordable prices, allowing more patients to receive the treatment.
Armed-T technology has another advantage, noted Dr. Chuang. The molecules that are attached to the surface of the T cell can be customized to allow the T cell to target and kill a specific type of cancer cell.
CytoArm has developed five Armed-T products — CTA-01, CTA-02, CTA-03, CTA-04 and CTA-06. Experiments on lab mice with human B-cell leukemia indicate that CTA-01 can rapidly target and effectively destroy B-cell leukemia cancer cells with no obvious signs of recurrence. CTA-03 is for treating prostate cancer and CTA-04 is for treating metastatic breast carcinoma.
CytoArm's first Armed-T product, CTA-02, is for treating incurable colorectal cancer associated with mutations in the epidermal growth factor receptor (EGFR) gene or its downstream effectors. In the first half of 2024, CytoArm is set to submit applications for CTA-02 clinical trials in Taiwan, Singapore, and the US.
Poised to become a powerful weapon against cancer, CytoArm's Armed-T technology received worldwide attention following its introduction. Dr. Chuang named Taiwan's maturing startup ecosystem a key contributor to CytoArm's success.
As a TMU-incubated startup, CytoArm developed a patent plan with TMU's guidance right when it was first founded. It also reached out to a U.S. IP firm for advice and confirmation that Armed-T does not infringe upon big pharma players' CAR-T patents, allowing the company to steer clear of obstacles that might hinder its research.
The industry, government, and academia are stepping up efforts to support innovations and startups. Since CytoArm's establishment in 2020, capital investment, professional guidance, and market-driven strategies have all fueled the company's growth. Going forward, Dr. Chuang suggests that the government strengthen research grant and talent incubation policies and drive the commercialization of academic research to keep Taiwan's industry reinvigorated.
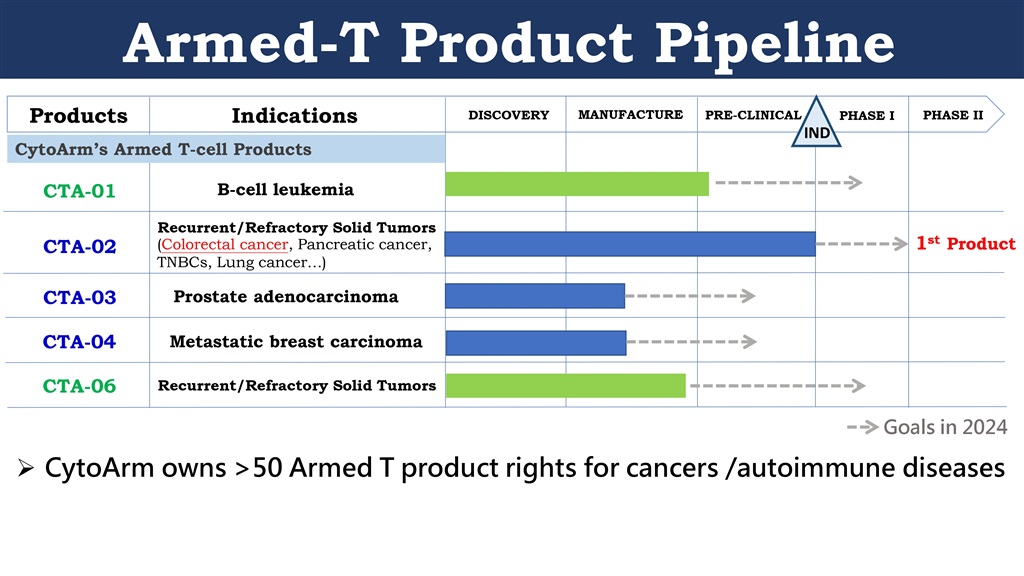
CytoArm's Armed-T products. The first product, CTA-02, is for treating incurable colorectal cancer associated with mutations in the EGFR gene or its downstream effectors. Phase I clinical trials are scheduled to begin in 2024. It can be used to treat pancreatic cancer, lung cancer, TNBC and other types of cancer in the future.



